This year the Ophthalmic Instrument Cleaning and Sterilization (OICS) Task Force issued a joint statement updating their recommended guidelines for sterilizing intraocular instruments. The following is a synopsis of the statement.
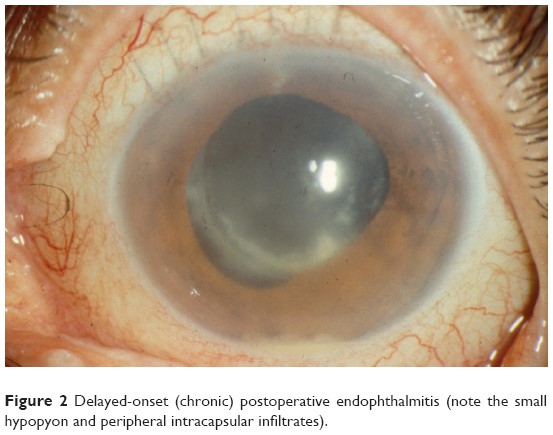
Postoperative infectious endophthalmitis and toxic anterior segment syndrome (TASS) are rare, but potentially sight-threatening complications of cataract and other intraocular surgery. The small volume of the eye and its sensitivity to minute amounts of chemical or microbial contaminants means that improper instrument cleaning or sterilization practices might pose a significant risk to patients.
Most of the recommended practices are derived from existing evidence-based recommendations for cleaning and sterilizing all surgical instruments in general, from published analyses of TASS outbreaks, and from manufacturers’ instructions for use (IFU) for surgical instruments and equipment. In addition, task force members have collaborated in performing new research that supports certain recommendations.
Toxic anterior segment syndrome is an acute severe inflammatory reaction to a toxic contaminant introduced into the anterior chamber during intraocular surgery. In addition to severe anterior chamber cell and flare, it might be associated with fibrin, hypopyon, diffuse limbus-to-limbus corneal edema, atonic pupil, secondary glaucoma, and in some cases, vitreous cells. Because of these signs, TASS might be misdiagnosed and mistreated as infectious endophthalmitis. Even if TASS resolves with treatment and without permanent sequelae, the patient often suffers the emotional trauma of believing he or she might have a potentially blinding infection.
All facilities should establish written protocols for instrument cleaning and sterilization. These “policies and procedures” should be based on industry standards and guidelines with input from the nursing and medical staff. Personnel involved should be properly trained in handling, cleaning, and sterilizing intraocular surgical instruments and subject to periodic oversight.
Cleaning and decontamination, which include thorough rinsing and flushing, should precede disinfection or sterilization. It is recommended that ophthalmic instrumentation should be cleaned separately from nonophthalmic surgical instruments. Contaminated and soiled instruments should also be cleaned in an area separate from where packaging and sterilization take place.
During decontamination and cleaning, all debris inclusive of ophthalmic viscosurgical device (OVD) should be removed from the instruments. It might be helpful to keep instruments moist until the cleaning process begins to avoid drying of debris and OVD.2,3,16,20 A dampened lint-free cloth or soft brush should be used to clean instruments in accordance with the manufacturer’s IFU. Additional or repeated cleaning and rinsing steps might be required on an instrument-by-instrument basis to ensure removal of all debris and OVD.
One practice that is controversial is the use of enzymatic detergents for decontaminating intraocular surgical instruments. The manufacturer’s IFU that accompany ophthalmic instruments and ultrasound cleaning baths often call for the use of enzymatic cleaners, the omission of which would therefore be considered off-label. However, the necessity of enzymatic detergents for cleaning contaminated intraocular instruments has not been established. Contrary to some manufacturers’ IFU for their intraocular instrument, it is our position that enzymatic detergents should not be routinely required for intraocular instruments for several reasons. These detergents typically contain subtilisin or alpha amylase exotoxins, neither of which is denatured by autoclave sterilization. Corneal endothelial toxicity from enzymatic detergents has been documented in both animal and human studies. Inappropriate use or incomplete rinsing of enzymatic detergents has been associated with outbreaks of TASS.
Studies have shown that while following the manufacturer’s IFU, even minute enzyme residue left on intraocular instruments can cause TASS. The small-diameter lumens and fragile nature of intraocular instruments often make complete removal of all traces of enzyme detergent difficult. Much larger enzyme residues are found if thorough rinsing is not performed.
Ultrasonic cleaning poses another risk factor for TASS according to the TASS Task Force surveys.
If an ultrasonic cleaner is used, the technician should remove all visible soil before placing instruments in the ultrasonic cleaner. The ultrasonic unit should be designated for cleaning medical instruments and preferably should only be used for ophthalmic instruments. If a unit is used for other types of surgical instruments, it should be emptied, cleaned, and rinsed before use with ophthalmic instruments to avoid cross contamination. Ultrasonic machines should be emptied, cleaned, disinfected, rinsed, and dried at least daily. Unless otherwise specified by the manufacturer, cleaning should be performed as per the machines IFU.
The method of instrument sterilization should be based on guidelines from the medical device, packaging system, and sterilizer manufacturer. Routine monitoring and verification of sterilizer function with biological indicators should be performed at least weekly, and preferably daily, in accordance with the sterilizer manufacturer’s IFU and documented in the facility log.
My advice? Do the research, check how many cases of TASS and endophthalmitis you have had at your facility. Talk to the vendor who supplies your instruments. Discuss with your CSSD Team the pro’s and cons of using enzymatic versus non enzymatic detergents. Review your policies for reprocessing Intraocular Surgical Instruments from pre cleaning at point of use, to decontamination and washing and wrapping terminal sterilization. Use Enhanced Visual Inspection on these delicate instruments. If an enzymatic detergent is used ensure that they are rinsed of all residue and OVA debris. If you have the ability to do residual protein testing you might consider including it as part of your QA procedures. Look at the possibility of using a non enzymatic detergent for these instruments. Each and every member of the sterile processing Team can play their part in ensuring that their patients receive the highest quality reprocessed instruments and that we do not put them at risk due to out dated practices. Their vision is in our hands.
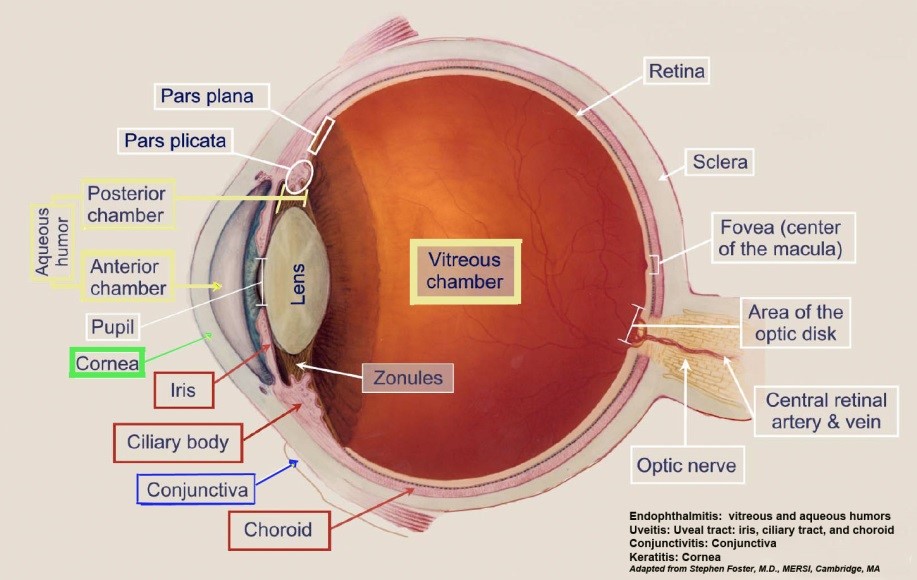
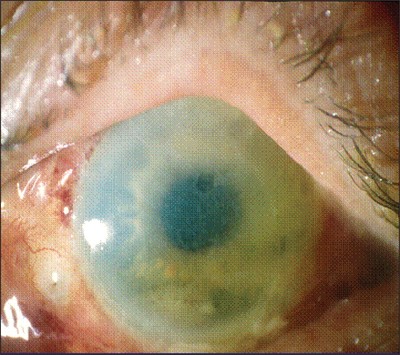
TASS
Enhanced Visual Inspection